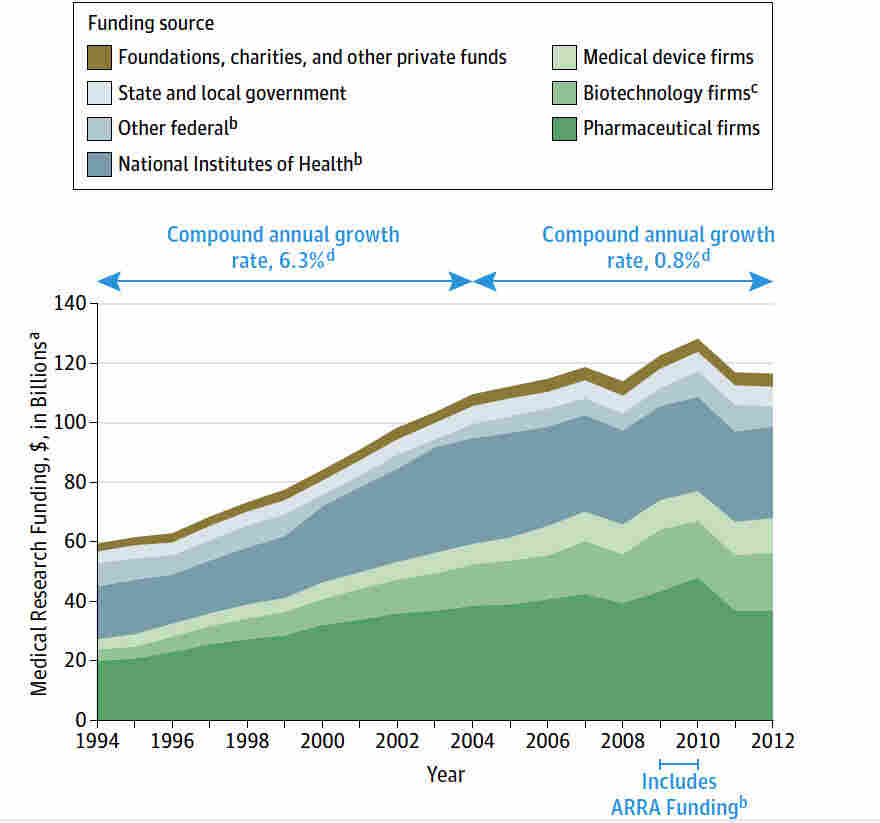
Medical research funding plays a crucial role in advancing healthcare and ensuring patient safety in research, yet it faces significant challenges. Recent funding cuts have raised concerns about the impact on collaborative research efforts, particularly at esteemed institutions like Harvard Medical School. With a halt in financial support, vital programs such as the SMART IRB, which oversees multisite studies, are threatened. These funding cuts not only impede the progress of important medical studies but also strain the institutional review board (IRB) oversight essential for protecting human research participants. Without adequate funding, the potential for setbacks in patient safety and welfare becomes alarmingly high.
Securing financial support for clinical research is indispensable for fostering innovation and upholding the ethical standards necessary in medical studies. The reduction in resources can severely hinder collaborative efforts among hospitals and research institutes, jeopardizing research outcomes and participant welfare. Enhanced fiscal backing is critical for maintaining a robust regulatory framework, such as IRB review, which is pivotal in safeguarding the interests of individuals involved in research trials. The landscape of scientific inquiry at prestigious universities, particularly Harvard, is intricately linked to the flow of funding, highlighting the interconnectedness of financial support and patient safety measures in the medica field. As such, a renewed focus on securing medical research funding is essential to ensure that scientific advancement does not come at the cost of participant protection.
Understanding the Importance of Medical Research Funding
Medical research funding is the backbone of innovative healthcare solutions and advancements in patient care. When funding is robust, it not only facilitates groundbreaking studies but also ensures the safety and rights of participants involved in these studies. In recent years, cuts to funding from federal sources, such as the National Institutes of Health (NIH), have initiated a serious disruption in this vital process. For example, the Trump administration’s freeze of over $2 billion in federal research grants to institutions like Harvard has significantly hampered ongoing research efforts. Without sufficient funding, several research initiatives related to patient safety begin to stagnate, impacting the quality and effectiveness of future medical solutions.
Moreover, the discontinuation of essential funding streams can lead to a ripple effect within the research community. Collaborative research efforts often hinge on joint funding agreements between various institutions, promoting shared expertise and resources, which in turn fosters comprehensive studies. When funding is cut, researchers and institutions are forced to abandon or significantly alter their projects. This disarray not only affects the immediate research but also dissuades potential future collaborations, limiting opportunities for innovation in medical science. Maintaining robust medical research funding is crucial for developing new treatments and ensuring comprehensive oversight for patient safety in research.
The Role of IRBs in Protecting Patients During Medical Research
Institutional Review Boards (IRBs) are essential players in the medical research landscape, primarily responsible for protecting the rights and welfare of research participants. They conduct meticulous reviews of research proposals, ensuring compliance with ethical standards and federal regulations. The responsibilities of IRBs encompass assessing research methodologies, risk management, and ensuring that informed consent is properly obtained and maintained. In recent discussions, the importance of IRB oversight has come to the forefront as funding cuts threaten to destabilize these critical review processes. Researchers must be vigilant in adhering to ethical guidelines, and without the oversight of IRBs, there is a pronounced risk of participants being put in harm’s way.
Additionally, the disruption of funding can severely undermine the operational capacity of IRBs. For instance, without sufficient resources, IRBs may struggle to provide thorough training and support to researchers, thereby affecting their ability to comprehend and adapt to emerging ethical challenges in research. This lack of operational support may lead to fewer safeguards for participants, raising serious ethical concerns about the integrity of clinical studies. The cumulative impact of reduced funding on IRB oversight can result not only in setbacks for current studies but also in a broader decline in public trust regarding the safety of engaging in medical research.
Impact of Funding Cuts on Collaborative Research Efforts
Collaborative research efforts are pivotal in advancing healthcare solutions, particularly in complex fields like oncology and neurology. However, funding cuts can critically impede these collaborative initiatives. The SMART IRB system was designed to streamline the review process for multi-site studies, significantly enhancing the efficiency of collaborative research. With the recent halt in funding at Harvard, the ability to expand this system has been severely restricted. New institutions are unable to participate in ongoing research, resulting in fewer resources and limited manpower to support comprehensive studies. This stagnation not only affects the timeline of research but potentially delays medical advancements that could have a significant impact on patient care.
Moreover, the ability to share knowledge and expertise between institutions is greatly hindered by funding cuts. When collaborative research projects are funded adequately, they foster interdisciplinary partnerships that can lead to innovative therapies. However, without these financial resources, many studies face personnel shortages, limited equipment access, and reduced participant recruitment capabilities. As collaborative research relies on a solid network of institutions, the consequences of funding cuts ripple across the entire biomedical research community, potentially delaying the next breakthrough treatment and harming future patient safety initiatives.
Ensuring Patient Safety Amidst Research Challenges
Patient safety is the cornerstone of any medical research endeavor, necessitating rigorous oversight and ethical commitment. During challenging times marked by funding cuts and administrative disruptions, the dedication to maintaining patient safety must remain unwavering. The IRB structure, a key player in ensuring participant protection, evolves in response to historical failures and ethical breaches in research. Regulatory frameworks were developed to safeguard the rights of participants, and these cannot be compromised in the face of financial constraints. As the landscape of research funding shifts, it becomes imperative for institutions to prioritize these safeguards to ensure that participants are treated ethically and their safety is uncompromised.
In the face of ongoing funding challenges, research institutions must adapt to ensure that patient safety remains a top priority. This includes seeking alternative funding sources, fostering community partnerships, and advocating for stronger policies that emphasize ethical oversight. By upholding robust safety standards and transparent communication with study participants, researchers can mitigate the risks associated with funding disruptions. Ultimately, the commitment to patient safety not only enhances the integrity of research but also rebuilds trust and confidence in the medical research community.
The Consequences of Stagnation in Medical Research Progress
The cessation of funding can lead to a troubling stagnation in medical research progress, which directly affects the development of new treatments and improvements in patient care. The freeze on federal research grants at institutions like Harvard signifies a broader crisis in the research community, where the momentum gained in groundbreaking studies can be abruptly halted. Delayed research projects mean that critical studies cannot progress to the next phases, resulting in lost opportunities to save lives or improve health outcomes. This creates a daunting gap in the availability of innovative therapies, fundamentally altering the trajectory of medical advancements.
Additionally, stagnation in research invites a range of socioeconomic consequences. Communities relying on advances in medical science for public health improvements may experience setbacks in treatment access or disease prevention strategies. Furthermore, there is a growing public skepticism regarding the efficacy and safety of participation in clinical trials, particularly when studies are interrupted or poorly managed due to funding shortages. This lessened public trust can deter individuals from enrolling in studies, leading to a significant reduction in participant eligibility and diversity, ultimately compromising the validity of research outcomes and hindering the continuous improvement of healthcare.
Navigating Ethical Dilemmas in Medical Research Funding Cuts
The intersection of ethics and funding in medical research presents a unique set of dilemmas, notably during times of budget constraints. As funding sources dwindle, the temptation may arise for researchers to cut corners, potentially compromising participant safety in pursuit of completing projects. This scenario raises significant ethical questions about the responsibilities researchers hold towards their participants. It becomes essential for institutions to maintain stringent oversight to prevent lapses in adherence to ethical guidelines, ensuring that the welfare of research subjects remains paramount, regardless of funding challenges.
Moreover, navigating these ethical dilemmas necessitates a collaborative dialogue within the research community. Institutions should promote transparency and ethical training, ensuring all researchers understand the implications of cutting costs on research integrity. Creating an open environment where ethical concerns can be discussed fosters a culture of accountability. By prioritizing ethics in the face of funding uncertainties, researchers can devise innovative solutions that do not hinder patient safety, thus reinforcing the ethical foundation of the medical research community despite financial setbacks.
The Future of Patient-Centric Research
The landscape of medical research is ever-evolving, particularly as we aspire to establish a more patient-centric approach. Future endeavors must prioritize the needs and safety of participants at all levels of research. Engaging patients in the research process, from design to implementation, empowers them and builds trust in the research community. Adequate funding is crucial for ensuring these mechanisms are in place, as it facilitates the necessary educational outreach and participatory frameworks that enhance patient involvement.
As we look forward, addressing the funding dynamics in medical research will be critical to advancing patient-centered methodologies. Innovations in budgeting, such as embracing public-private partnerships, could provide alternative funding solutions to safeguard patient interests. Furthermore, emphasizing transparency in how funds are allocated and utilized will promote accountability and encourage collaborative research efforts that align with the health interests of the community. Building a sustainable future for patient-centric research hinges on securing the necessary resources while maintaining robust ethical guidelines.
Historical Context of Medical Research Ethics
To understand the importance of medical research ethics, one must consider the historical context that shaped today’s standards. Past atrocities, such as the Tuskegee Syphilis Study and unethical experimentation during wartime, have facilitated overarching changes in how ethical considerations are integrated into research protocols. These historical lessons emphasize the necessity for thorough oversight, particularly from IRBs, in safeguarding vulnerable populations. As we face contemporary challenges such as funding cuts, it is imperative to remember these historical failings and continue striving for an ethical framework that emphasizes patient welfare.
Moreover, the evolution of regulatory policies in response to past ethical breaches illustrates the dynamic nature of medical research regulations. The establishment of IRBs and the implementation of stringent guidelines were direct reactions to historical abuses, ensuring that participants’ rights are kept at the forefront of research practices. As stakeholders, researchers today carry the responsibility to uphold these ethical principles, advocating for policies that prevent past mistakes from recurring, even in the face of funding challenges. By reflecting on these pivotal moments in history, we can better appreciate the importance of ethical conduct in advancing medical research.
Building Trust Through Transparent Research Practices
Trust between researchers and participants is paramount in the conduct of medical research. Transparent research practices—ensuring that participants are fully informed about the purpose, risks, and benefits of studies—are essential to uphold this trust. With funding cuts, there’s an increased pressure to rush processes, which can hinder clear communication and mislead participants about the nature of the studies. Institutions must prioritize transparency even in difficult financial times to ensure that participants feel valued and respected throughout the research process.
Furthermore, fostering trust requires institutions to engage with communities actively. By establishing clear channels of communication and involving community members in research discussions, researchers can gain a deeper understanding of participants’ concerns. Building a culture of trust is vital, especially as the landscape of medical research undergoes shifts due to funding issues. Researchers who take the time to cultivate transparency and build relationships within their communities significantly enhance participant engagement, ensuring better outcomes for all involved.
Frequently Asked Questions
How does medical research funding impact patient safety in research?
Medical research funding is crucial for ensuring patient safety in research. It allows for thorough oversight by Institutional Review Boards (IRBs) that evaluate research proposals to protect participants’ rights and welfare. Adequate funding enables IRBs to conduct reviews, ensure compliance with regulations, and monitor studies effectively, thus improving patient safety.
What would be the effects of funding cuts on patient safety in medical research?
Funding cuts can severely disrupt the oversight of medical research, risking patient safety. With fewer resources, IRBs may struggle to review studies and monitor ongoing research adequately. This can lead to lapses in patient protection, increased risks of harm, and diminished public trust in research initiatives.
How does collaborative research efforts benefit from stable medical research funding?
Stable medical research funding facilitates collaborative research efforts by supporting multi-site studies that require coordinated oversight. It allows multiple institutions to work together under a single IRB, streamlining the process and improving efficiency while ensuring patient safety and regulatory compliance across all sites.
What is the role of the IRB in ensuring safety for patients involved in medical research funded by NIH?
The IRB plays a critical role in safeguarding patients in NIH-funded medical research. They review and monitor studies to ensure ethical practices, informed consent, and risk mitigation, ultimately enhancing the safety and rights of research participants throughout the duration of the study.
Why are federal funding cuts considered harmful to patient safety in medical research?
Federal funding cuts create significant obstacles for patient safety in medical research. They hinder the operations of IRBs and halt ongoing studies, which can lead to unsafe conditions for participants and undermine the integrity of clinical research, ultimately affecting public health outcomes.
How has Harvard medical research been affected by funding cuts regarding patient safety?
Harvard medical research has faced substantial disruptions due to funding cuts, particularly relating to the SMART IRB program, which oversees multi-site studies. Such interruptions compromise the framework for ensuring patient safety and ethical conduct, threatening the continuation of crucial research efforts.
What challenges arise from the impact of funding cuts on medical research regarding IRB oversight?
Funding cuts challenge IRB oversight by limiting available resources for thorough reviews and monitoring. This can delay the approval of research studies, hinder collaboration, and ultimately compromise the safety and well-being of participants involved in medical research.
| Key Points | Details |
|---|---|
| Funding Cuts | The Trump administration’s freeze of over $2 billion in federal research grants has disrupted numerous medical research initiatives. |
| Impact on Patient Safety | Cuts hinder the oversight provided by Institutional Review Boards (IRBs), essential for protecting the rights and safety of research participants. |
| Role of IRBs | IRBs review research proposals to ensure compliance with regulations and monitor risks to participants’ welfare. |
| Historical Context | Past medical abuses highlighted the need for ethical research oversight and protective measures for participants. |
| Ongoing Support | Despite funding freezes, Harvard Medical School is supporting ongoing collaborative research efforts crucial for patient safety. |
Summary
Medical research funding is crucial for ensuring the safety and rights of patients involved in clinical studies. The recent cuts in federal funding have led to significant disruptions in oversight processes, threatening the welfare of research participants. With Institutional Review Boards facing operational challenges, there is a growing concern over the potential harms to both participants and the integrity of the research enterprise. Ongoing support from institutions like Harvard Medical School is vital in maintaining collaborative efforts and safeguarding patient interests in the face of these funding challenges.