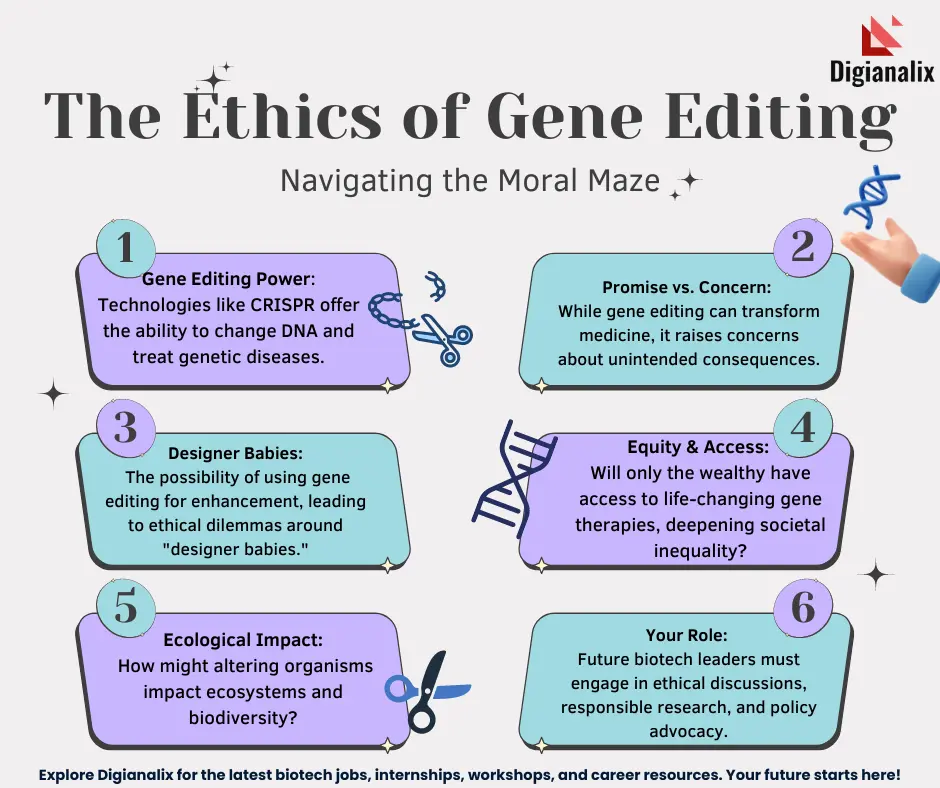
Gene editing ethics is an increasingly vital topic in contemporary bioethics, raising critical questions about our ability to manipulate human genetics through technologies like CRISPR. As advancements allow for genetic modifications aimed at curing diseases such as sickle cell anemia, the ethical implications cannot be ignored. Should we intervene in the natural course of human life, or is there a responsibility to preserve our genetic diversity? These dilemmas pose significant challenges, particularly regarding health equity and access to such innovative treatments, which often come at exorbitant costs. Ultimately, while the promise of CRISPR technology offers potential solutions, it also compels us to reevaluate our moral compass regarding genetic intervention.
The discourse surrounding genetic modification encompasses a range of ethical considerations that impact societal norms and individual rights. With the advent of advanced technologies for gene editing, we face profound inquiries about the implications of changing inherent human traits. The potential for interventions in genetics, especially for conditions like sickle cell disease, prompts a critical examination of the responsibilities we bear towards future generations. As we explore this terrain, terms such as bioethics and health justice emerge, reflecting the need for equitable access and comprehensive oversight in the application of genetic therapies. In navigating these complex questions, we must carefully weigh the consequences of our technological capabilities against the backdrop of human dignity and diversity.
The Promise of CRISPR Technology in Medicine
CRISPR technology has opened new frontiers in the realm of medical science, particularly in the fight against hereditary diseases such as sickle cell anemia. This revolutionary gene editing tool allows for precise modifications to the genome, providing the potential to correct genetic mutations that lead to debilitating conditions. For instance, the ability to alter somatic cells means that patients can benefit from treatments that previously seemed impossible. By directly targeting the genes responsible for diseases, CRISPR stands as a beacon of hope for countless individuals and families affected by these conditions.
Moreover, the potential applications of CRISPR extend beyond just curing diseases. Researchers envision using this technology to prevent genetic disorders altogether by making changes at the germline level. This could mean that future generations might inherit a version of their DNA that has been ‘edited’ to eliminate predispositions to certain conditions. However, as we explore these new possibilities, we must also consider the challenges associated with such profound changes in our genetic make-up, particularly concerning ethical boundaries and the implications of such interventions.
Ethics and Gene Editing: Key Concerns
The advent of CRISPR technology brings forth significant ethical questions, essential to the ongoing discourse in bioethics. Specifically, the ability to edit genes, especially in germline cells, raises difficult moral dilemmas. For instance, should we change traits that are not life-threatening, such as those found in individuals with Down syndrome? This prompts crucial discussions about the limitations that should be placed on gene editing and who gets to make these decisions. The potential for ‘designer babies’ based on parental preferences adds yet another layer of ethical complexity to gene modification.
Furthermore, the socio-economic implications of gene editing must not be overlooked. The high costs associated with CRISPR treatments, such as the estimated $2.2 million for sickle cell intervention, bring forth questions of health equity. Who benefits from these advanced therapies, and who gets left behind? As highlighted by health professionals, innovation in genetics may inadvertently deepen existing health disparities unless equitable access is ensured. The need for comprehensive frameworks that prioritize both innovation and ethical considerations is more pressing than ever.
The Intersection of Health Equity and Genetic Modification
Health equity is a crucial aspect in the dialogue surrounding gene editing technologies like CRISPR. When groundbreaking treatments, such as those for sickle cell anemia, are priced beyond the reach of many, the disparities in healthcare accessibility become glaring. There is a profound responsibility to ensure that advancements in genetic modification are not only available to affluent populations but are equitably distributed across all social strata. This represents not just a public health issue but also a moral imperative to create systems that prevent exacerbating existing health inequalities.
Addressing health equity within the context of genetic modification also involves considering the diverse populations that may be affected by such technologies. Are regulatory bodies taking into account the varying needs and values of different communities? As we advance in gene editing capabilities, there’s a pressing need for inclusive discussions that contemplate cultural contexts and variations in health beliefs. Ensuring that all voices are heard in the development and implementation of these technologies is essential in building a future where health equity is a reality, not just an aspiration.
Cautionary Tales in the Use of CRISPR
While the promise of CRISPR technology is immense, cautionary tales exist that warrant careful consideration. The ease with which gene editing can alter human DNA poses significant risks not only to individual patients but potentially to the human gene pool as a whole. For example, if indiscriminate use of CRISPR leads to unintended genetic consequences, we might witness negative repercussions that extend beyond the intended benefits of disease prevention. The story of gene editing is not just one of potential cures—it’s also one that involves the uncharted territory of unforeseen genetic interactions and implications.
Moreover, the ethical ramifications of potential genetic enhancements raise further concerns. Imagine a scenario where parents, influenced by personal desires or societal pressures, opt to edit their child’s traits—whether for intelligence, athleticism, or even aesthetics. This notion of ‘playing God’ disrupts the natural variability that defines human existence. Therefore, it becomes imperative that as we stride forward with technological innovations like CRISPR, there are strict ethical guidelines to ensure that such powerful tools are used responsibly, preserving the essence of human diversity.
Future Perspectives on Gene Editing
As we look ahead, the future of gene editing and CRISPR technology holds incredible promise alongside significant challenges. The discussions initiated by thought leaders like Neal Baer concerning the ethical implications of gene editing must continue to shape our approach. As advancements in genetic engineering evolve, integrative frameworks that encompass both scientific innovation and ethical rigor will be key to guiding policy-making and clinical practices. By doing so, we can harness the full potential of CRISPR technology while navigating the ethical landscape responsibly.
Moreover, stakeholder engagement, including patients, healthcare professionals, and ethicists, will be crucial in shaping the future of gene editing. Collaborative discussions and research can help ensure that innovations are aligned with societal values and help address concerns around health equity and access. By fostering a proactive dialogue, we can envision a future where gene editing enriches the human experience rather than complicating it—making it possible for equitable advancements in health for all.
Gene Editing and Unintended Consequences
The implementation of gene editing technologies like CRISPR is not without its share of unintended consequences. While the potential to eradicate diseases such as sickle cell anemia is groundbreaking, the long-term outcomes of such genetic alterations can be unpredictable. For example, while editing genes to reduce LDL cholesterol may seem beneficial, it is essential to understand the complex interactions these genes have within the body and their evolutionary significance. Disrupting educated guesses can lead to alternative health issues that might arise years down the line, complicating the narrative around gene editing.
Consequently, thorough research followed by robust regulatory frameworks is essential to mitigate the risk associated with gene editing. We must change our approach to scientific research, emphasizing not only discovery but also consequences. Larger dialogues that engage scientists, ethicists, and the public can help bridge the gap between innovation and accountability, ensuring that the advancements we make in gene editing do not come at the cost of overall health stability.
Policy Implications of Gene Editing Technologies
The rapid advancement of gene editing technologies such as CRISPR necessitates a reevaluation of current healthcare policies. Policymakers must consider not only the therapeutic potentials but also the ethical implications and socioeconomic dimensions of these technologies. Establishing regulations that promote responsible use of gene editing can help foster public trust while preventing misuse or abuse of these revolutionary tools. This includes developing eligibility criteria for treatments and ensuring accessibility for underserved populations, aligning with principles of health equity.
Furthermore, there is a need for continuous dialogue among regulatory bodies, healthcare providers, and ethicists to establish comprehensive guidelines that evolve alongside our understanding of gene editing technologies. These discussions are vital in ensuring that policies not only prioritize innovation but also advocate for ethical considerations and equitable access. By taking a proactive stance, we can create a framework that supports the advancement of these technologies while safeguarding the rights and health of individuals affected by them.
Balancing Innovation with Social Responsibility
As we delve deeper into the capabilities of gene editing technologies, striking a balance between innovation and social responsibility becomes increasingly crucial. While the scientific community may be eager to explore new frontiers, innovators and researchers must remain cognizant of the societal implications of their work. It is not enough to create therapies that can save lives; we must also contemplate how these advancements impact diverse communities and the ethical ramifications of our choices.
Implementing a model of accountability in gene editing practices involves listening to various stakeholders, including patients, advocacy groups, and ethicists. By ensuring that the voices of those impacted by genetic modifications are included in dialogues surrounding innovation, we can support a future where health technologies are developed responsibly and equitably. In doing so, we can create a healthcare landscape where advancements benefit all individuals, upholding the ideals of fairness and social justice.
The Role of Public Perception in Gene Editing
Public perception plays a vital role in the acceptance and implementation of gene editing technologies. Misinformation and misunderstandings about gene editing can adversely affect public trust and support for these innovations. Educational campaigns that accurately represent the science behind CRISPR and address ethical concerns are essential to fostering an informed society. Engaging the public in discussions about the implications and benefits of gene editing can promote transparency and encourage responsible dialogue.
Moreover, understanding public sentiment can guide the development of regulatory frameworks that reflect societal values and expectations. Policymakers and researchers must prioritize communication efforts that demystify gene editing technologies and facilitate meaningful engagement with communities. By doing so, we not only cultivate acceptance but also empower individuals to voice their concerns and perspectives on the use of these advanced biotechnologies, ensuring a more inclusive and equitable approach to future innovations.
Frequently Asked Questions
What are the ethical implications of using CRISPR technology for treating genetic diseases like sickle cell anemia?
The ethical implications of using CRISPR technology for treating genetic diseases, such as sickle cell anemia, include considerations of health equity, informed consent, and the moral responsibility to alter human genetics. As CRISPR technology provides the ability to edit both somatic and germline genes, it raises questions about who decides which conditions warrant treatment, how to ensure accessibility to these advancements, and the potential long-term impacts on the human gene pool.
How does gene editing raise concerns about health equity in treatments like CRISPR for sickle cell anemia?
Gene editing, particularly with CRISPR technology, raises significant concerns about health equity as the high costs associated with such treatments, like the estimated $2.2 million for curing sickle cell anemia, create disparities in access. These disparities can exacerbate existing inequalities in healthcare, leaving vulnerable populations without access to life-saving treatments. Ethical frameworks must account for fairness and justice to ensure equitable distribution of gene-editing therapies.
In what ways does gene editing challenge traditional bioethics in managing human traits?
Gene editing challenges traditional bioethics by questioning the morality of altering traits that are not strictly pathological, such as choosing genetic attributes for non-life-threatening conditions. This raises complex debates about parental rights, the nature of diverse human experiences, and the implications of defining what constitutes a ‘normal’ or desired human trait versus those that require medical intervention, particularly in genres of genetic modification.
What are the potential risks of unintended consequences in gene editing technologies like CRISPR?
The potential risks of unintended consequences in gene editing technologies like CRISPR include unpredictable genetic interactions and alterations. For instance, while CRISPR can successfully target specific diseases, unintended edits may disrupt other important genetic functions, leading to unforeseen health issues. The long history of gene evolution suggests a complex interplay that is not fully understood, necessitating rigorous testing and ethical oversight before widespread application.
Should consent for genetic modification be required, especially in cases affecting unborn children?
Yes, consent for genetic modification should be a requisite ethical consideration, particularly regarding unborn children. The use of CRISPR technology to modify germline genes raises profound ethical dilemmas about who decides the traits and future of a child. Informed consent processes must ensure that parents are fully aware of the implications and responsibilities of making such decisions on behalf of their children, respecting the notion of autonomy and individual rights.
How can bioethics guide the application of CRISPR technology in society?
Bioethics can guide the application of CRISPR technology in society by establishing frameworks that prioritize informed consent, equity in access, and the burden of proof for safety and efficacy. It emphasizes the responsibility to consider not only the potential benefits of genetic modifications but also the broader social implications, including human rights, justice, and the diversity of human conditions, ensuring the technology serves the greater good.
What role does oversight play in the ethical use of CRISPR and gene editing technologies?
Oversight plays a crucial role in the ethical use of CRISPR and gene editing technologies by ensuring compliance with legal, ethical, and safety standards. It involves monitoring research practices, clinical applications, and potential misuse, including concerns about genetic modifications for non-therapeutic enhancements. Effective regulatory frameworks are needed to prevent abuses while fostering innovation responsibly.
| Key Point | Description |
|---|---|
| Introduction to Gene Editing Ethics | Discussion on the ethical implications of gene editing technology, particularly CRISPR, which can change human genetics. |
| Potential Benefits of CRISPR | CRISPR can potentially cure genetic diseases like sickle cell anemia, giving hope for treatment options that did not exist before. |
| Ethical Questions Raised | It raises critical ethical questions about what constitutes a disease worth curing and who decides which genes can be edited. |
| Cost and Accessibility Issues | The high cost of gene therapies (e.g., $2.2 million for sickle cell) raises issues of health equity and accessibility. |
| Human Rights and Genetic Modification | Modification of genes for non-medical reasons (e.g., enhancing abilities) questions parental rights and human diversity. |
| Regulatory and Oversight Challenges | Concerns about oversight in countries where ethical regulations may not be strictly enforced. |
| Unintended Consequences of Gene Editing | Gene modification may lead to unforeseen effects due to the complex interactions of genes. |
Summary
Gene editing ethics is a complex and critical discussion about the moral implications of altering human genetics. As advancements in technologies like CRISPR offer potential cures for genetic disorders, they simultaneously challenge our understanding of what it means to be human. Questions arise about the fairness of access to such therapies, the wisdom of making genetic modifications, and the risks of unintended consequences. In considering gene editing, it is essential to navigate the balance between innovation and ethical responsibility to ensure that we respect human diversity and prevent misuse of such powerful technologies.